Delta G And Keq Relationship
Chapter 18. Chemic Thermodynamics
Energy under Nonstandard Weather
Jessie A. Key
- To be able to determine free free energy at nonstandard atmospheric condition using the standard alter in Gibbs free energy, ΔK°.
- To empathize and utilise the relationship between ΔK° and the equilibrium constant 1000.
Many reactions do not occur under standard weather, and therefore nosotros demand some ways of determining the free energy under nonstandard conditions.
Using Standard Change in Gibbs Gratuitous Energy, ΔOne thousand°
The alter in Gibbs energy under nonstandard conditions, ΔG, can be determined from the standard change in Gibbs complimentary energy, ΔGrand°:
![]()
where R is the platonic gas constant 8.314 J/mol 1000, Q is the reaction quotient, and T is the temperature in Kelvin.
Under standard atmospheric condition, the reactant and production solution concentrations are one M, or the pressure of gases is 1 bar, and Q is equal to 1. Taking the natural logarithm simplifies the equation to:
![]()
Nether nonstandard conditions, Q must exist calculated (in a manner similar to the adding for an equilibrium constant). For gases, the concentrations are expressed as partial pressures in the units of either atmospheres or confined, and solutes in the units of molarity.
For the reaction ![]() :
:
![]()
Consider the following reaction:
4NHiii(chiliad) + 5Oii(grand) ⇌ 6H2O(g) + 4NO(g)
- Use the thermodynamic data in the appendix to calculate ΔG° at 298 M.
- Calculate ΔGrand at 298 K for a mixture of 2.0 bar NHthree(g), 1.0 bar O2(g), 1.5 bar H2O(g), and 1.2 bar NO(g).
Solution
The Relationship betwixt ΔG° and One thousand
In that location is a direct relationship between ΔG⁰ and the equilibrium abiding K. We can plant this relationship by substituting the equilibrium values (ΔOne thousand = 0, and Yard = Q) into the equation for determining free energy change under nonstandard weather:
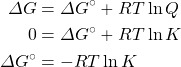
We at present have a fashion of relating the equilibrium constant directly to changes in enthalpy and entropy. Every bit well, nosotros can now determine the equilibrium constant from thermochemical information tables or determine the standard free energy change from equilibrium constants.
The K sp for CuI(s) at 25°C is ane.27 × 10−12. Determine ΔGrand° for the following:
Cu+(aq) + I−(aq) → CuI(southward)
Solution
![]()
The equation given is in the contrary direction to the definition of K sp:
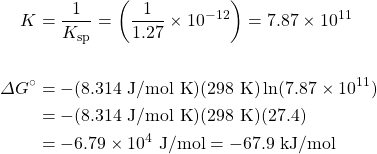
- The complimentary energy at nonstandard conditions tin exist determined usingΔG = ΔG° + RT lnQ.
- There is a direct relationship between ΔG° and the equilibrium abiding 1000:ΔYard° = −RT lnK.
Delta G And Keq Relationship,
Source: https://opentextbc.ca/introductorychemistry/chapter/free-energy-under-nonstandard-conditions/
Posted by: gordonfastir.blogspot.com

![Rendered by QuickLaTeX.com \begin{align*} \Delta G^{\circ}&=\underset{\text{products}}{\sum n\Delta G^{\circ}{}_{\text{f}}}-\underset{\text{reactants}}{\sum m\Delta G^{\circ}{}_{\text{f}}} \\ &=[(6\times -228.6\text{ kJ/mol})+(4\times 87.6\text{ kJ/mol})] \\ &\phantom{=}-[(4\times -16.4\text{ kJ/mol})+(5\times 0.0\text{ kJ/mol})] \\ &=(-1021.2\text{ kJ/mol})-(-65.6\text{ kJ/mol}) \\ &=-955.6\text{ kJ/mol}=-9.56\times 10^5\text{ J/mol} \end{align*}](https://opentextbc.ca/introductorychemistry/wp-content/ql-cache/quicklatex.com-a309419db4bd9e197f743f54c40df8b9_l3.png)
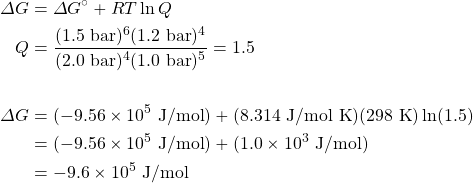

0 Response to "Delta G And Keq Relationship"
Post a Comment